
In a groundbreaking move, the U.S. Food and Drug Administration (FDA) has granted approval for the first-ever treatment specifically designed for children diagnosed with generalized myasthenia gravis (gMG). This historic decision marks a turning point in pediatric neuromuscular care, offering young patients new hope in managing this debilitating autoimmune disease.
Table of Content:-
Understanding Generalised Myasthenia Gravis (gMG)
Generalised myasthenia gravis (gMG) is a chronic disorder in which the body's immune system mistakenly targets the neuromuscular junctions—areas where nerves signal muscles to move. This misdirected immune response leads to the accumulation of harmful antibodies, resulting in muscle weakness, fatigue, and difficulty with everyday tasks such as walking, talking, and even breathing in severe cases. Until now, treatment options for children with gMG have been extremely limited, making this approval particularly significant.
The Role of Soliris in Treating gMG
The newly approved medication, Soliris (eculizumab), is a monoclonal antibody therapy that works by blocking a specific protein in the immune system responsible for neuromuscular damage. By preventing the overactivation of the immune response, Soliris effectively reduces the symptoms of gMG and improves overall muscle function. Previously approved for adults with gMG and other blood and immune system disorders, Soliris now becomes the first targeted therapy available for children aged six and older diagnosed with this condition.
Also Read: Living Near Drains Could Increase Cancer Risk, Warns ICMR – Here’s What You Need to Know!
A Scientific Breakthrough Backed by Research
The FDA's approval follows extensive clinical research, including trials conducted in adults with gMG and subsequent safety evaluations in pediatric patients. According to the study, children aged 12 to 17 who received Soliris exhibited similar safety and efficacy results as adults, with the most commonly reported side effects being muscle and bone pain. These findings demonstrate that the treatment is both effective and well-tolerated in younger populations, paving the way for broader use among pediatric patients.
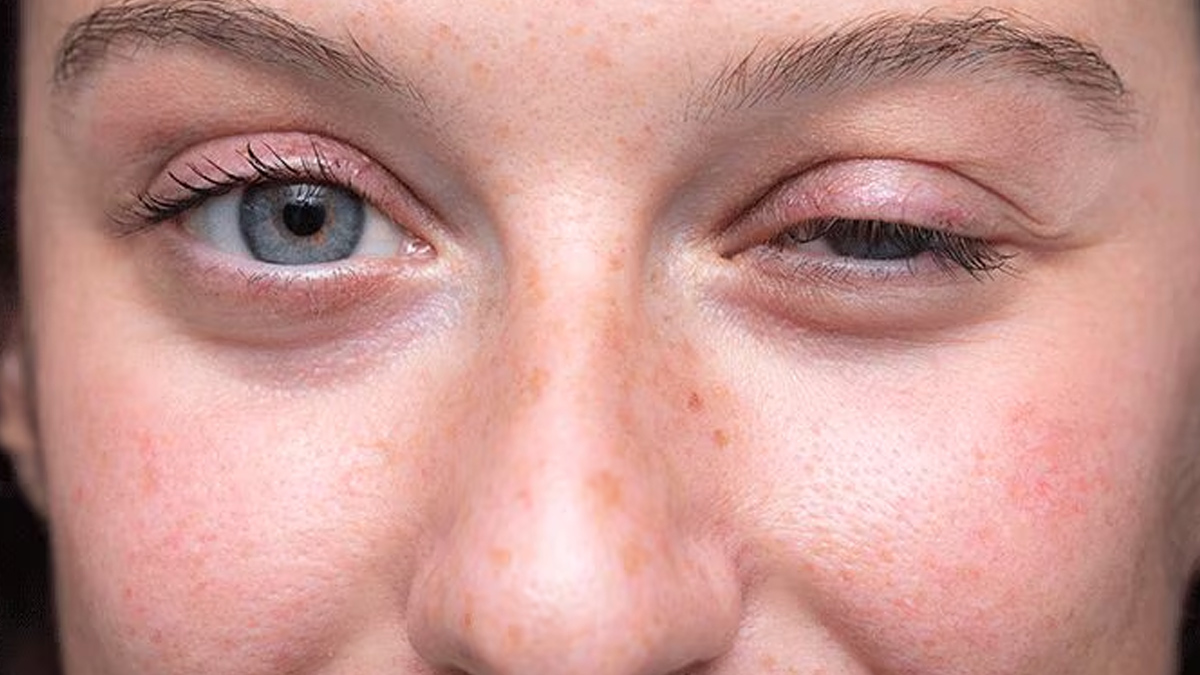
Expert Insights on the Approval
Medical experts and patient advocacy groups have hailed this decision as a transformative step in neuromuscular disease treatment. Dr. Sharon Hesterlee, Chief Research Officer at the Muscular Dystrophy Association, emphasised the significance of the approval, stating, "This is a monumental advancement in the management of pediatric myasthenia gravis. It brings much-needed relief and optimism to families navigating this complex condition."
Also Read: Delhi Sees Surge In Influenza B, H1N1 Cases Across Age Groups, Say Doctors
Administration and Safety Precautions
Soliris is administered intravenously (IV) over a period ranging from 35 minutes for adults to 1 to 4 hours for children. Due to its mechanism of action, the drug is only available through a Risk Evaluation and Mitigation Strategy (REMS) program, as it can increase the risk of serious infections, particularly meningococcal infections. To mitigate this risk, patients must receive a meningococcal vaccination at least two weeks before starting treatment.
What This Means for Children with gMG
For children struggling with gMG, the approval of Soliris represents a major leap forward in treatment accessibility and effectiveness. The ability to target the underlying immune dysfunction rather than just managing symptoms offers young patients a chance at improved mobility, independence, and overall quality of life.
Bottomline
While the approval of Soliris is a milestone, it also highlights the ongoing need for continued research into pediatric neuromuscular disorders. The success of this treatment underscores the importance of innovation in developing targeted therapies for rare diseases, ultimately improving healthcare outcomes for affected children.
The FDA’s decision signals a new era in pediatric care—one where cutting-edge treatments are no longer reserved solely for adults but are extended to the youngest and most vulnerable patients. As research advances, further breakthroughs in treating myasthenia gravis and similar disorders are expected, providing greater hope for families worldwide.
https://www.onlymyhealth.com/living-near-drains-could-increase-cancer-risk-warns-icmr-12977827113
Also watch this video
How we keep this article up to date:
We work with experts and keep a close eye on the latest in health and wellness. Whenever there is a new research or helpful information, we update our articles with accurate and useful advice.
Current Version