
The U.S. Food and Drug Administration (FDA) has granted approval for Zepbound, a weight-loss medication, to treat moderate to severe obstructive sleep apnea (OSA) in individuals with obesity. This decision marks a significant milestone in addressing one of the most common sleep disorders affecting millions of Americans.
Table of Content:-
Zepbound: A Dual-Purpose Solution
Zepbound, developed by pharmaceutical giant Eli Lilly, belongs to the GLP-1 receptor agonists class, a family of drugs that includes Ozempic. Initially approved in November 2023 for weight management in obese or overweight individuals, Zepbound’s new indication highlights its broader therapeutic potential. The drug is designed to be used alongside a reduced-calorie diet and increased physical activity, offering a comprehensive approach to managing obesity and sleep apnea.
Dr. Sally Seymour, director of the FDA’s Division of Pulmonology, Allergy, and Critical Care, lauded the approval as a “major step forward for patients with obstructive sleep apnea.”
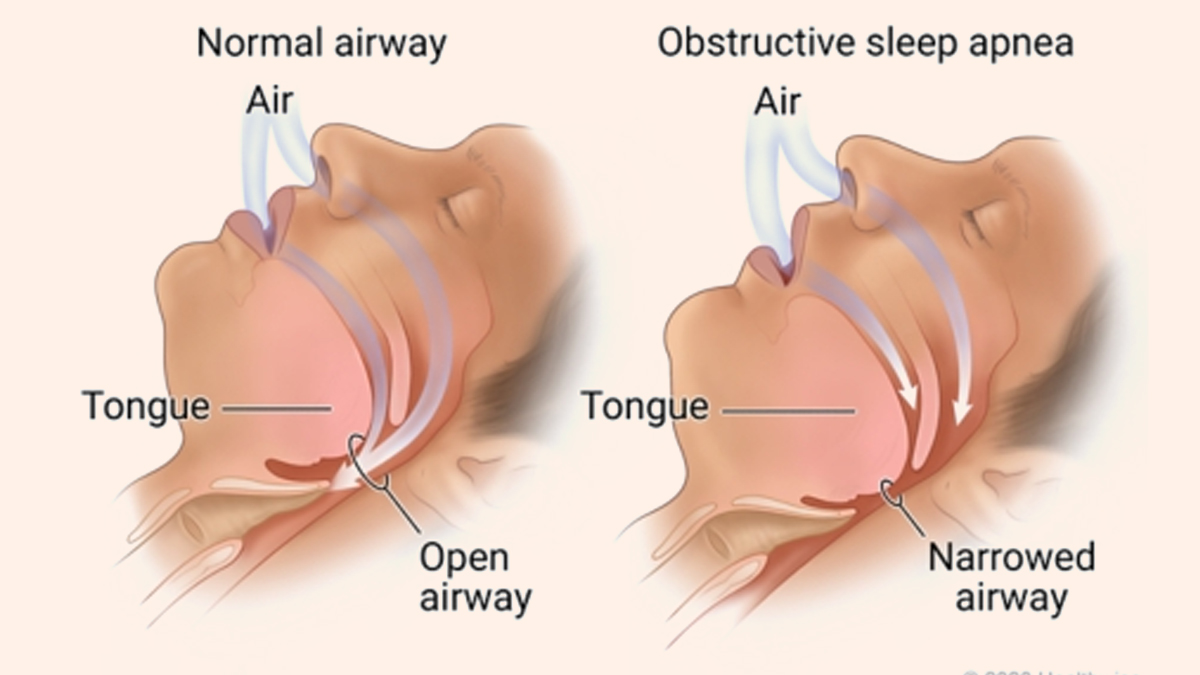
The Link Between Obesity and Obstructive Sleep Apnea
Obstructive sleep apnea is characterized by repeated interruptions in breathing during sleep due to airway blockages, often caused by excessive tissue in the throat. According to the American Academy of Sleep Medicine, OSA affects up to 30 million individuals in the U.S. and is closely associated with obesity. Symptoms such as snoring, gasping for air during sleep, and excessive daytime fatigue can lead to severe complications, including cardiovascular and neurological risks.
Weight loss is a proven strategy to alleviate the severity of OSA. Eli Lilly’s clinical trials reveal that nearly half of Zepbound users experienced significant improvements, with some no longer exhibiting symptoms of the disorder.
Also Read: Shark Tank India Sparks Debate: Namita Thapar and Anupam Mittal Face Off on Work-Life Balance
Clinical Trial Insights: Effectiveness Beyond Expectation
The FDA’s approval of Zepbound for OSA is based on two extensive clinical trials involving approximately 470 participants. In these studies, the drug’s effectiveness was measured by the apnea-hypopnea index (AHI), which tracks the frequency of breathing disruptions per hour during sleep.
In one trial, where participants were not using continuous positive airway pressure (CPAP) machines, Zepbound reduced the AHI by an average of 25 events per hour, compared to a reduction of about five in the placebo group. In another trial involving CPAP users, the reduction was even more substantial, with Zepbound decreasing events by 29 per hour versus six for the placebo.
Participants also saw dramatic weight loss, averaging 18-20% of their body weight — equivalent to about 45-50 pounds over a year. This weight reduction was accompanied by improvements in blood pressure and reduced inflammation, mitigating cardiovascular risks commonly linked with OSA.
Addressing Accessibility and Costs
While Zepbound’s approval is a breakthrough, affordability remains a concern. Without insurance, the medication costs approximately $1,060 per month. However, Eli Lilly offers financial assistance through discounts, coupons, and a more affordable vial version that requires manual injection.
Also Read: Epigamia CEO Rohan Mirchandani Dies Of Cardiac Arrest At 42: Know Possible Causes In Your 40s
Medicare currently limits coverage for obesity medications unless they serve other medical purposes, such as reducing cardiovascular risks. However, this approval could pave the way for expanded insurance coverage for OSA-related treatments.
Managing Side Effects
Most side effects of Zepbound were mild to moderate gastrointestinal issues, occurring primarily during the initial dosing period. Researchers emphasized that these effects were manageable and outweighed by the drug’s benefits in reducing OSA symptoms and improving overall health.
A Paradigm Shift in OSA Treatment
The approval of Zepbound underscores the evolving landscape of OSA management, combining weight-loss benefits with improved respiratory health. Patrik Jonsson, president of Lilly Cardiometabolic Health, expressed optimism about the drug’s impact, highlighting its potential to reduce the burden of untreated OSA and improve patient outcomes.
As Zepbound becomes available for this new indication, its role in addressing the interconnected challenges of obesity and sleep apnea may redefine treatment strategies for millions struggling with these conditions.
Also watch this video
Read Next
Athiya Shetty Debuts Her Baby Bump, Amid BGT Series: At What Month Do Mothers Get A Baby Bump?
How we keep this article up to date:
We work with experts and keep a close eye on the latest in health and wellness. Whenever there is a new research or helpful information, we update our articles with accurate and useful advice.
Current Version